|
Instituut voor Studie van Schimmel in Menselijke
Woningen |
|
Institut pour l'Etude de Moisissure Fongique dans
Habitations Humaines |
|
|
|
Forschung Insitute für Schimmelpilze in
Innenräumen |
|
MYCOLOGICAL INSTITUTE
|
|
for the study
of |
|
FUNGAL MOLD
IN HUMAN HABITATIONS |
|
|

Should Dogs be Used to Inspect for Toxic
Mold? |
© Copyright
and/or publishing rights held by the Canadian Veterinary Medical
Association
Noninvasive Intranasal Infusion
in dogs with Nasal Aspergillosis
Jimmy H. Saunders, Luc Duchateau,
Christophe Stark, and Henri van Bree
Departments of Medical
Imaging (Saunders, van Bree) and Physiology, Biochemistry and
Biometrics (Duchateau), Faculty of Veterinary Medicine, Ghent
University, Salisburylaan 133, 9820 Merelbeke, Belgium; Departement
of Clinical Sciences of Companion Animals, Faculty of Veterinary
Medicine, University of Lige, Sart Tilman, B44, 4000 Lige
Belgium |
|
|
|
|
Abstract |
|
Computed tomography (CT) was performed on 36 dogs with
nasal aspergillosis to assess whether this imaging technique
can be used to predict the success of a noninvasive intranasal
infusion of enilconazole. A CT score based on the severity of
the disease was given to each dog, prior to treatment, by
dividing the nasal cavities and frontal sinuses into 8
anatomical regions. After therapy, the dogs were classified
into 2 response groups (success group: dogs cured after 1
treatment; failure group: dogs needing more than 1 treatment
or with treatment failure). No significant relationship on the
logistic scale was found between the CT score and the response
to treatment. High sensitivity (treatment failures correctly
predicted) and specificity (treatment successes correctly
predicted) could not be obtained at the same time, whatever
the cut-off value chosen. The results of this study suggest
that CT cannot predict the therapeutic success of nasal
aspergillosis in dogs treated with a 1-hour infusion of
enilconazole. However, dogs with a low score seem to be good
candidates to respond after 1 treatment.
|
| |
|
Introduction |
| |
|
Mycotic infection is a common cause of chronic nasal
disease in the dog (1).
Aspergillus
fumigatus , an ubiquitous soil saprophyte,
is the most common causative agent (1).
Effective treatment of this disease is still challenging.
Systemic administration of thiabendazole, ketoconazole, or
fluconazole resulted in clinical cure in approximately 50% of
dogs, and of itraconazole in 70% of dogs (2,3,4,5,6).
Topical administration of antifungals (twice daily for 7 to 14
d) by surgically implanted drains into the frontal sinuses,
the nasal cavities, or both was effective in 77% to 89% of
dogs treated with either clotrimazole or enilconazole (7,8).
Recently, a noninvasive technique using nonsurgically placed
catheters has been developed to infuse the topical drug into
the nasal cavities and frontal sinuses under general
anesthesia (8,9).
Prior to treatment, the nasal cavities are isolated by
occluding the nasopharynx and nares with Foley catheters (8,9).
Then, an antifungal is instilled into the nasal cavities and
left “in situ” for a period of 1 h (8,9).
This technique results in a more complete distribution of the
drug and is associated with fewer complications than is a
topical treatment through surgically placed catheters (8,9).
It was associated with a clinical cure in 47% to 65% of dogs
after 1 treatment and 87% after repeated treatments with
either clotrimazole or enilconazole (8,9).
Currently, several treatment protocols with variations of this
technique have been under investigation to improve therapeutic
success, tolerance by the animal, and compliance by the owners
(9,10,11,12,13,14).
Relapse at a later date is very unusual for dogs in which
fungal elimination has been confirmed by a reexamination (1).
A grading system based on clinical, radiographic, and
rhinoscopic examinations has been used to evaluate the
severity of the lesions before treatment and the clinical cure
after treatment (4).
The technique of computed tomography (CT) is becoming more
available in veterinary medicine and is widely used for
examination of head disorders, including the evaluation of the
nasal cavities and associated structures (15,16,17,18,19,20,21,22,23,24).
Computed tomography offers several advantages relative to
conventional radiography for examination of the nasal cavities
and frontal sinuses: cross-sectional images that eliminate the
superimposition of different structures, adjustment of the
contrast scale to optimize optical density and discriminate
even the fine turbinate structures, multiplanar
reconstructions for better evaluation of the cribriform plate,
and, with helical CT, the possibility to perform examinations
under deep sedation instead of general anesthesia (24,25,26).
Not surprisingly, CT has been shown to be more sensitive than
radiography in detecting nasal disease and defining of the
extension of the lesions (16,19,20).
In dogs with nasal aspergillosis the efficacy of the
distribution of an antifungal into the nasal cavities and
frontal sinuses when using a noninvasive technique has been
demonstrated by CT (27).
Computed tomography has been used, in both human and
veterinary medicine, in attempts to predict the outcome of
multiple disease processes on the basis of an accurate
evaluation of the extension of the lesions, particular CT
features, scoring or scaling systems, or perfusion studies (28,29,30,31).
Mathews et al (8)
used CT in 32 dogs with nasal aspergillosis to try to predict
the likelihood of a successful treatment by using a scoring
system based on the severity of the lesions. A cut-off point
was determined at which a sufficiently high sensitivity and
specificity could be obtained. They concluded that their
scoring system could be used by others to evaluate CT images
of dogs with nasal aspergillosis and to inform owners about
the likelihood of a favorable response to treatment (8).
The purpose of this study was to evaluate the value of CT
to predict the effect of therapy in 36 dogs with nasal
aspergillosis treated with a noninvasive infusion of
enilconazole
1%. |
| |
|
Materials and
methods
|
| |
|
Thirty-six dogs were used in this study. All tests made on
these dogs were part of the routine clinical examination in
dogs with chronic nasal disease and were performed with the
owners consent. Fourteen different breeds were represented:
rottweiler (n = 9), golden retriever (n = 6),
Labrador retriever (n = 5), Belgian shepherd dog
(n = 3), Newfoundlander (n = 2), Afghan hound
(n = 2), German shepherd dog (n = 2), Doberman
pinsher (n = 1), Alaskan malamute (n = 1),
basset hound (n = 1), bull terrier (n = 1),
pointer (n = 1), Pyrenean shepherd dog (n = 1),
and teckel (n = 1). The mean age of the dogs was 4.4 y.
There were 19 males and 16 females. Physical, serologic,
imaging (radiography and CT), and rhinoscopic examinations
were carried out for all dogs. During rhinoscopy, swabs,
cytobrush, and biopsies were obtained for culture, cytologic
examination, and histologic examination, respectively.
Diagnosis of nasal aspergillosis was based on at least 3
positive diagnostic tests, including direct visualization of
fungal colonies at rhinoscopy.
Computed tomography was performed with a 4th generation
helical CT (Picker 6000 PQ; Picker, Eastlake, Ohio USA).
General anesthesia was induced with droperidol and fentanyl
(Thalamonal; Janssen-Cilag, Beerse, Belgium), 0.08 mg/kg body
weight (BW), IV, and penthotal (Phenobarbital; Abbott, Abbott
Park, Illinois USA), 5 to 15 mg/kg BW, IV, and then maintained
with halothane 1.5% to 2% (Fluothane; Zeneca, Wilmington,
Delaware USA). All dogs were placed in ventral recumbency.
Transverse contiguous slices were obtained from the caudal
level of the frontal sinuses to the nostrils. Technical
settings were 110 kV, 125 mA, pitch 1.5, slice thickness 3 mm.
Pre- and postcontrast (700 mgI/kg BW, IV, of a nonionic iodine
contrast medium) (Omnipaque 300; Nycomed, Brussels, Belgium)
studies were performed. Reformatted dorsal plane images were
also obtained. Hard copies were printed with a bone window
(window width (WW) 3500 — window level (WL) 500) and a
soft-tissue window (WW 340 — WL 25). When necessary, the WW
and WL were adjusted on the computer monitor for visualization
of other structures.
The CT studies of these 36 dogs were scored retrospectively
by a board-certified radiologist (JHS). The same scoring
system as described by Mathews et al (8)
was used to score the nasal cavities and frontal sinuses.
Therefore, each nasal cavity was divided in 4 anatomical
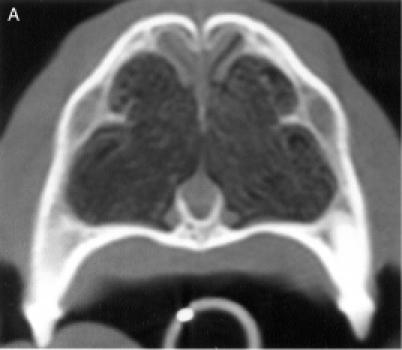
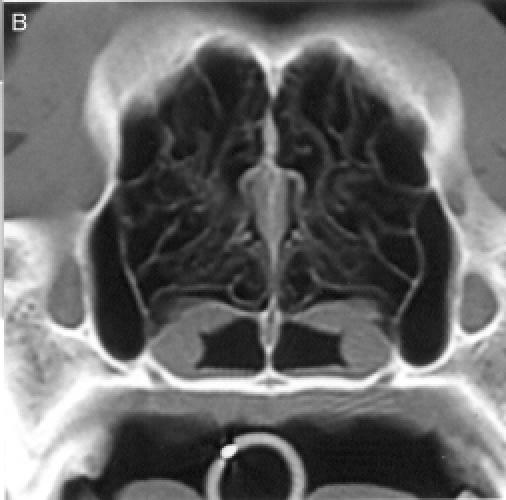
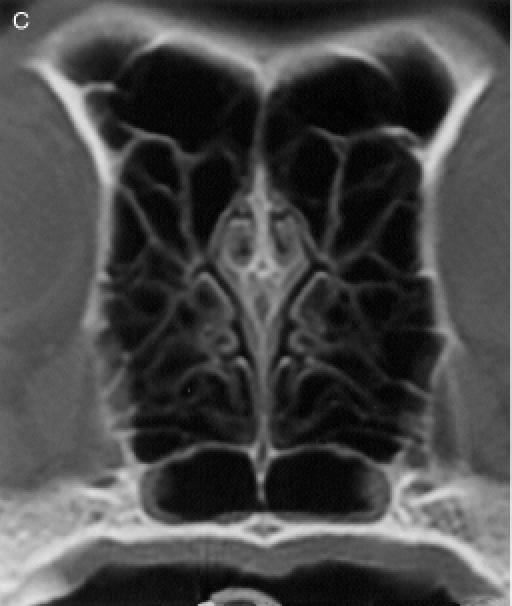
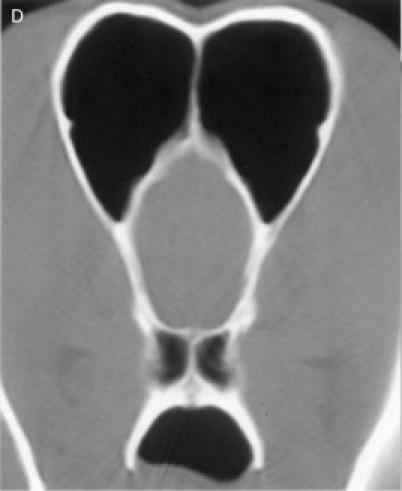
regions, for a total of 8 anatomic sites: region I — the nasal
turbinates rostral to the maxillary recess, region II — the
maxillary turbinates at the level of the maxillary recess,
region III — the ethmoid turbinates caudal to the maxillary
recess, and region IV — the frontal sinus (Figure
1). Each of the 8 anatomic sites was given a score of 0 to
3 based on the severity of the lesions (0 = no abnormality, 1
= mild turbinate atrophy or fluid accumulation, 2 = moderate
disease, 3 = severe disease). For the frontal sinus (region
IV), periorbital invasion and frontal bone changes
(hyperostosis/lysis/mixed) were also used to evaluate the
severity of the disease. The scores were added to provide a
total score for each dog. Maximum possible total CT score was
24.
All dogs were treated with a 1-hour infusion of 1% (10
mg/mL) enilconazole (Imaverol; Janssen-Cilag), delivered via
nonsurgically placed catheters by using a technique comparable
with that described by Mathews et al (21).
The dogs were evaluated clinically and rhinoscopically after 3
to 4 wk and the treatment was repeated until clinical and
rhinoscopic healing was achieved. The dogs were classified
into 2 response groups on the basis of their response to
treatment (success group: dogs cured after 1 treatment;
failure group: dogs cured after more than 1 treatment or not
cured).
A logistic regression analysis, using the CT score as a
continuous independent variable and treatment success or
failure as a dependent variable, was performed to evaluate the
relationship between the CT scores and the probability of
failure. Both total score and score of the 4 deepest locations
(regions III and IV) were used, multiplying the latter by 2 in
order to work on the same scale. A receiver operating
characteristic (ROC) curve was derived connecting pairs of
sensitivity (percentage of dogs with an unfavorable response
to treatment that were predicted to be treatment failures) and
specificity (percentage of dogs with a favorable response to
treatment that were predicted to do so) for different cut-off
values of the CT score (32).
Special attention was focused on the results obtained with a
CT score < 8, which was defined in a previous study as a
cut-off value (8). |
| |
|
Results |
| |
|
The dog's breed, sex, and age, as well as its CT score and
number of treatments, are reported in Table
1. From the 36 dogs, there were 20 dogs that were cured
after 1 treatment (success group) and 16 dogs that were cured
after more than 1 treatment or were considered treatment
failures (failure group). There was no statistically
significant relationship between CT scores and probability of
treatment failure, either for total score (P = 0.12) or
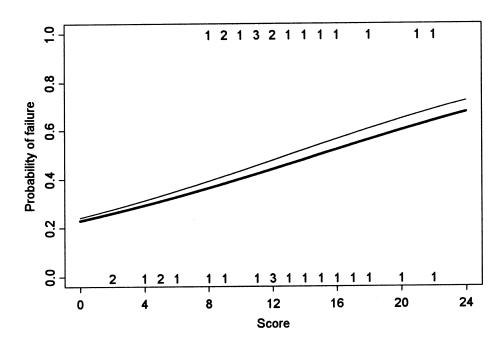
for the score of the 4 deepest locations (P = 0.06) (Figure
2). The failure probability increased slightly with the
score of the 4 deepest locations as compared with the total
score. Dogs with a CT score < 8 were all successes, and it
is mainly due to these dogs that there was a positive,
although nonsignificant, relationship between the CT score and
the failure probability.
The ROC curve demonstrates that high sensitivity and
specificity cannot be obtained at the same time, whatever the
cut-off value chosen. When using a cut-off value of < 8,
the sensitivity is equal to 100% (exact 95% CI: 79% to 100%),
but the specificity is low and equal to 30% (exact 95% CI: 12%
to 54%). To increase the specificity to 80%, the cut-off CT
score value needs to be increased to 16, but then the
sensitivity goes below 20%.
All dogs (6/6) that had a score < 8 were cured after 1
treatment. From the dogs with a score ≥ 8, clinical cure was
obtained in 46.6% (14/30) after 1 treatment; in 53.4% (16/30),
clinical cure required more than 1 treatment or was not
obtained. | | |
|
Discussion |
| |
|
In the present study, the severity of the lesions as
assessed by CT could not be related to treatment failure (more
than 1 treatment). These results may be explained by the
difficulty in relating the CT features of nasal aspergillosis
with the pathophysiology of the disease. The CT score was
based on the severity of the lesions, expressed mainly in
terms of the amount of abnormal soft tissue and the degree of
turbinate destruction. The amount of abnormal soft tissue in a
diseased nasal cavity can be evaluated grossly by CT, but, in
most cases, CT does not allow the nature of the tissue to be
defined, and it is often even difficult to differentiate soft
tissue from fluid (21,24).
Use of contrast studies may permit differentiation between the
mucosa and other soft tissue or fluid, or between necrotic and
vascularized soft tissue. However, it has been demonstrated
that attenuation measurements are susceptible to a variety of
errors in a diseased nasal cavity, due mainly to the presence
and sometimes mixing of many complex structures of different
physical densities (21,24,33).
In this study, the postcontrast CT studies were not helpful
in evaluating the extension of the lesions. On the one hand, a
dilution with a large volume of debris, necrotic tissue,
exudate, inflammatory tissue, or large granulomas in the nasal
cavity or frontal sinus may complicate optimal diffusion of
the antifungal medication (21).
Moreover, this soft tissue may serve as a growth medium for
the fungus (21).
However, even in such cases, a single topical infusion may be
successful. On the other hand, an empty nasal cavity does not
guarantee that the treatment will be effective, as there may
be squamous or osseous metaplasia of the mucosa that decreases
diffusion of the antifungal medication or “mucosal stripping”
with compromised local circulation and immunity that will
prevent healing (34,35).
The impact of the degree of turbinate destruction on the
outcome is also difficult to predict. A severe turbinate
destruction, emptying the nasal cavity, will permit better
diffusion of the antifungal medication (21).
However, a too severe turbinate destruction may diminish the
local immunity and prevent healing (34).
Other CT features that were used to evaluate the severity
of the disease in our classification system were invasion of
periorbital structures, cribriform plate destruction, and
frontal bone changes. Fungal infection of periorbital
structures may be a cause of treatment failure, and it has
been suggested that the topical therapy should be combined
with a systemic antifungal medication in these dogs (7,36).
Periorbital involvement was present in 2 dogs in our study.
Both dogs were treated successfully by a topical antifungal
medication after 1 and 3 treatments, respectively. The effect
of the leakage of an antifungal medication into the central
nervous system in case of cribriform plate destruction has not
been studied in dogs with nasal aspergillosis (21).
In the present study, 1 dog that showed localized cribriform
plate destruction was clinically cured after 2 topical
infusions of 1% enilconazole without clinical evidence of
neurological signs. Bony changes are not considered to
influence therapeutic success.
The failure to relate the severity of the lesions on CT to
treatment failure may also be explained by the inability of CT
to give information about potentially important predictors of
therapeutic success, such as the environmental status, a
bacterial or fungal secondary infection, an impaired immune
function, or the resistance of the fungus to the antifungal
medication (7,34,36).
The results of the present study are in contradiction with
those obtained by Mathews et al (8).
In the present study, no significant relationship could be
found between CT score and treatment failure (more than 1
treatment needed), while in the previous study, a cut-off
point of 8 allowed good classification with respect to
sensitivity and specificity. With a cut-off point of 8, a high
sensitivity (100%) was obtained in the present study compared
with the Mathews study (71% and 78%) (8).
However, the specificity (30%) was very low compared with that
obtained by Mathews et al (79% and 93%) (8).
Thus, using a cut-off point < 8, only 6 of the 20 dogs that
needed only 1 treatment were predicted to do so. On the basis
of the present study, it appears that the choice of a cut-off
< 8 optimizes the sensitivity, while neglecting the
specificity. This means that the owner of a dog with a score
< 8 can be told that his dog will probably need only 1
treatment. For dogs with a score ≥ 8, no information can be
given to the owner, as almost half the dogs (46%) needed only
1 treatment.
The difference in results between the 2 studies could be
attributed to differences in the use of the scoring system,
differences in the treatment methods, the results, or both.
The CT criteria used to score the nasal cavities in each
region from 0 to 3, based on turbinate atrophy and fluid
accumulation, are not objectively measurable, thus preventing
an excellent agreement between reviewers from being obtained,
as was also observed in a previous study on CT of chronic
nasal disease (24).
In the study of Mathews et al (8),
significant differences between reviewers were found in 3 of
the 8 anatomic regions, but these differences did not have an
impact on the cut-off point. Thus, it cannot be excluded that
the observer in our study assigned higher scores overall.
However, even the use of other cut-off points did not permit
good sensitivity and specificity to be obtained at the same
time in our study.
Differences between the treatment methods include the
choice of the antifungal (enilconazole instead of
clotrimazole), intranasal debridement prior to treatment, and
placement of catheters in the frontal sinuses. According to
the literature, the results obtained with enilconazole are
comparable with those obtained with clotrimazole (8,9).
A large amount of soft tissue is present in the nasal
passages, in approximately 50% of the dogs with nasal
aspergillosis, and around the sinonasal ostium, in
approximately 70% (24).
The efficacy of an endoscopic curretage could not be evaluated
objectively in the present study, as the CT examinations were
performed only before rhinoscopic debridment of the nasal
cavity and frontal sinuses. However, when using enilconazole
that is active in its vapor phase for distances up to 10 mm,
the importance of endoscopic debridement and placement of
drains in the frontal sinus may contribute to a better
distribution of the drug, particularly in the mucosa (37).
Endoscopic placement of catheters in the frontal sinus has
been associated with an increased success rate (12).
However, the treatment results in our study are comparable
with those obtained by Mathews et al (8).
Consequently, the treatment method cannot be responsible for
the difference in results observed between the 2 studies.
A limitation of the present study is that only 6 dogs had a
score < 8. In all these dogs, this score was associated
with lesions restricted to the rostral half of the nasal
cavity, unilaterally in 3 dogs and bilaterally in 3 dogs,
while no dog had a frontal sinus infection without a
concurrent abnormality in the nasal cavity. Lesions restricted
to the nasal passages are encountered in approximately 25% of
the dogs with nasal aspergillosis (24).
All these dogs were correctly predicted to respond after 1
treatment. Dogs with a score < 8, therefore, seem to be
good candidates to respond after 1 treatment, but this has to
be confirmed on a larger number of dogs with a low CT score.
Except for the dogs with a score < 8, use of a larger study
will probably not modify the conclusions. It cannot be
excluded that a statistically significant relationship between
CT score and the probability of treatment failure may be
found. However, it probably would have little clinical
relevance. Firstly, it would only be a weak relationship
mainly due to the dogs with a CT score lower than 8 that were
all treatment successes. Secondly, for the current data, the
specificity was only equal to 33%. This value might change
slightly but not dramatically with a larger sample size due to
random variation, but the overall conclusions would not
actually change.
The results of this study showed no significant
relationship between severity of the lesions, as assessed by
CT, and results of therapy in 36 dogs treated with a
noninvasive intranasal infusion of 1% enilconazole. Potential
reasons are the difficulty in relating the CT features of
nasal aspergillosis to the pathophysiology of the disease, and
the inability of CT to give information about some important
predictors of treatment outcome. Consequently, the view that
it is possible on the basis of CT only, to predict treatment
outcome, appears to be too
simplistic.
Address all correspondence and reprint
requests to Dr. J.H.
Saunders |
| References |
- Sharp NJH, Harvey CE, Sullivan M. Canine nasal aspergillosis
and penicilliosis. Compend Contin Educ Pract Vet 1991;25:41–46.
- Harvey CE. Nasal aspergillosis and penicilliosis in
dogs: Results of treatment with thiobendazole. J Am Vet
Med Assoc 1984;184:48–50. [PubMed]
- Sharp NHJ, Burrell MH, Sullivan M, Cervantes-Olivares
RA. Canine nasal aspergillosis: serology and treatment
with ketoconazole. J Small Anim Pract 1984;25:149–158.
- Sharp NJH, Sullivan M. Use of ketoconazole in the treatment
of canine nasal aspergillosis. J Am Vet Med Assoc 1989;194:782–786.
[PubMed]
- Sharp NHJ, Harvey CE, O'Brien JA. Treatment of canine
nasal aspergillosis/penicilliosis with fluconazole (UK-49,858).
J Small Anim Pract 1991;32:513–516.
- Legendre AM. Antimycotic drug therapy. In : Bonagura
JD, eds. Kirk's current veterinary therapy XII small animal
practice. Philadelphia: WB Saunders, 1995:327–331.
- Sharp NHJ, Sullivan M, Harvey CE, Webb T. Treatment
of canine nasal aspergillosis with enilconazole. J Vet
Int Med 1993;7:40–43.
- Mathews KG, Davidson AP, Koblik PD, et al. Comparison
of topical administration of clotrimazole through surgically
placed versus non-surgically placed catheters for treatment
of nasal aspergillosis in dogs: 60 cases (1990–1996) J
Am Vet Med Assoc 1998;213:501–506.
- Zonderland JL, Störk CK, Saunders JH, Hamaider AJ, Balligand
MH, Clercx CM. Intranasal infusion of enilconazole for
treatment of sinonasal aspergillosis in dogs. J Am Vet
Med Assoc 2002; 221:1421–1425. [PubMed]
- Caulkett N, Lew L, Fries C. Upper-airway obstruction
and prolonged recovery from anesthesia following intranasal
clotrimazole administration. J Am Anim Hosp Assoc 1997;33:264–267.
[PubMed]
- Bray JP, White RAS, Lascelles BDX. Treatment of canine
nasal aspergillosis with a new non-invasive technique:
failure with enilconazole. J Small Anim Pract 1998;39:223–226.
[PubMed]
- McCullough SM, McKiernan BC, Grodsky BS. Endoscopically
placed tubes for administration of enilconazole for treatment
of nasal aspergillosis in dogs. J Am Vet Med Assoc 1998;212:67–69.
[PubMed]
- Smith SA, Andrews G, Biller DS. Management of nasal
aspergillosis in a dog with a single non-invasive intranasal
infusion of clotrimazole. J Am Anim Hosp Assoc 1998;34:487–492.
[PubMed]
- Ford RB. Canine nasal aspergillosis. Proc Annu Meet
Br Small Anim Vet Assoc 2000:90.
- Sackman JE, Adams WH, McGavin MD. X-ray computed tomography-aided
diagnosis of nasal adenocarcinoma, with extension to the
skull and central nervous system, in a dog. J Am Vet Med
Assoc 1989;194:1073–1076. [PubMed]
- Thrall DE, Robertson ID, McLeod DA, Heidner GL, Hoopes
J, Page RL. A comparison of radiographic and computed
tomographic findings in 31 dogs with malignant nasal cavity
tumors. Vet Radiol 1989;30:59–66.
- Koblik PD, Berry CR. Dorsal plane computed tomographic,
imaging of the ethmoid region to evaluate chronic nasal
disease in the dog. Vet Radiol 1990;31:92–97.
- Burk RL. Computed tomographic imaging of nasal disease
in 100 dogs. Vet Radiol Ultrasound 1992;33:177–180.
- Park RD, Beck ER, LeCouteur RA. Comparison of computed
tomography and radiography for detecting changes induced
by malignant neoplasia in dogs. J Am Vet Med Assoc 1992;201:1720–1724.
[PubMed]
- Codner EC, Lurus AG, Miller JB. Comparison of computed
tomography with radiography as a noninvasive diagnostic
technique for chronic nasal disease in dogs. J Am Vet
Med Assoc 1993;202:1106–1110. [PubMed]
- Mathews KG, Koblik PD, Richardson EF, Davidson AP, Pappagianis
D. Computed tomographic assessment of noninvasive intranasal
infusions in dogs with fungal rhinitis. Vet Surg 1996;25:309–319.
[PubMed]
- Schwartz T. Die rolle der Röntgendiagnostik und der
computertomographie in der diagnostik klinischer rhinitiden
des hundes unter besonderer berûcksichtigung von tumoren
und mykosen der nasen- und nasennebenhöhlen. Dissertation
zur Erlangung des Grades eines Doktors der Veterinârmedizin
an der Freien Universitât Berlin. Berlin. 1997; Journal
No 1986.
- Forrest LJ. The head: Excluding the brain and orbit.
Clin Tech Small Anim Pract 1999;14:170–176. [PubMed]
- Saunders JH, Zonderland JL, Clercx C, et al. Computed
tomographic findings in 35 dogs with nasal aspergillosis.
Vet Radiol Ultrasound 2002;43:5–9. [PubMed]
- Drost WT, Love NE, Berry CR. Comparison of radiography,
myelography and computed tomography in the evaluation
of canine vertebral and spinal cord tumors in sixteen
dogs. Vet Radiol Ultrasound 1996;37:28–33.
- Davidson AP, Mathews KG, Koblik PD, Theon A. Diseases
of the nose and nasal sinuses. In: Ettinger SJ. Textbook
of Veterinary Internal Medicine. 5th ed. Philadelphia:
WB Saunders, 2000:1003–1025.
- Richardson EF, Mathews KG. Distribution of topical agents
in the frontal sinuses and nasal cavity of dogs: comparison
between current protocols for treatment of nasal aspergillosis
and a new noninvasive technique. Vet Surg 1995;24:476–483.
[PubMed]
- Gillepsie MB, O'Malley BW Jr, Francis HW. An approach
to fulminant fungal rhinosinusitis in the immunocompromissed
host. Arch Otolaryngol Head Neck Surg 1998;124:520–526.
[PubMed][Full Text]
- Gielen I, van Bree H, Van Ryssen B, DeClercq T, DeRooster
H. Radiographic, computed tomographic and arthroscopic
findings in 23 dogs with osteochondrosis of the tarsocrural
joint. Vet Rec 2002;150:442–447. [PubMed]
- Wakai T, Shirai Y, Nomura T, Nagakura S, Hatakeyam K.
Computed tomographic features of hepatocellular carcinoma
predict long-term survival after hepatic resection. Eur
J Surg Oncol 2002;28:235–242. [PubMed][Full Text]
- Wang PC, Chu CC, Liang SC, Tai CJ. Outcome predictors
for endoscopic sinus surgery. Arch Otolaryngol Head Neck
Surg 2002;126:154–159.
- Hanley JA, McNeil BJ. The meaning and use of the area
under a receiver operating characteristic (ROC) curve.
Radiology 1982;143:29–36. [PubMed]
- Williams G, Bydder GM, Kreel L. The validity and use
of computed tomography attenuation values. Br Med Bull
1980;36:279–287. [PubMed]
- Pavletic MM, Clark GN. Open nasal cavity and frontal
sinus treatment of chronic canine aspergillosis. Vet Surg
1991;20:43–48. [PubMed]
- Sharp NHJ, Sullivan M, Harvey CE. Treatment of canine
nasal aspergillosis. In: Pract 1992:14,27–31.
- Willis AM, Martin CL, Stiles J. Sino-orbital aspergillosis
in a dog. J Am Vet Med Assoc 1999;214:1644–1647. [PubMed]
- Van Gestel J, Van Cutsem J, Thienpont D. Vapor phase
activity of imazalil. Chemotherapy 1981;27:270–276. [PubMed]
|
| |